CRISPR Nobel Likely to Promote Advanced Therapies Development
October 11, 2020 Diana Gershtein, M.Sc, MBA ,Cell Therapy Section ManagerCRISPR Nobel Likely to Promote Advanced Therapies Development
October 11, 2020
Diana Gershtein, M.Sc, MBA ,Cell Therapy Section Manager
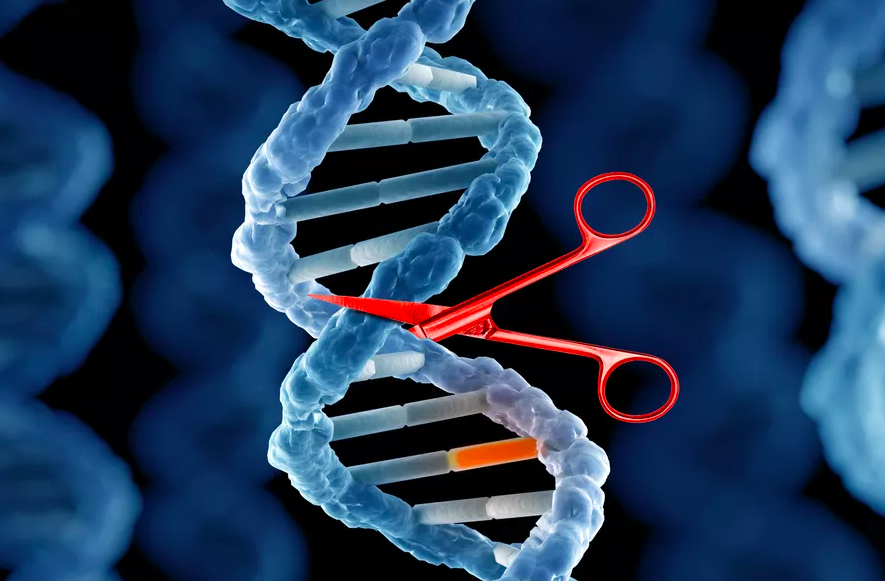
The Nobel Prize for Chemistry was awarded this year for the invention of Genetic Scissors: a tool for rewriting the code of life.
Emmanuelle Charpentier and Jennifer A. Doudna have discovered one of gene technology’s sharpest tools: the CRISPR/Cas9 genetic scissors. Using these, the DNA of animals, plants and microorganisms can be changed/edited with extremely high precision.
This technology has had a revolutionary impact on the life sciences, is contributing to new cancer therapies and may make the dream of curing inherited diseases come true.

Since the discovery of CRISPR/Cas9, the research of this tool has led to a blooming landscape of pre-clinical and clinical studies in humans.
FDA considers any use of CRISPR/Cas9 gene editing in humans to be gene therapy, thus requiring extensive regulatory efforts in order to bring such products from early concept to clinical application.
Gene therapy products are regulated by the FDA’s Center for Biologics Evaluation and Research (CBER). Clinical studies of gene therapy in humans require the submission of an investigational new drug application (IND) prior to their initiation in the United States, and marketing of a gene therapy product requires submission and approval of a biologics license application (BLA).
At Gsap, our team of Advanced Therapies experts is excited to be at the frontier of this field, with a unique portfolio of process development, preclinical, clinical and regulatory services, assisting our clients to bring gene-therapy products from early POC to realization into clinical use.
If you develop a gene-editing product, do not hesitate to contact us!
This Newsletter Prepared by:

Diana Gershtein, M.Sc., M.B.A.
Cell Therapy Section Manager
For more information about our services visit: