Extracellular Vesicles and Secretome – the rise of cell-free cell-derived products
May 19, 2022 Diana Gershtein MSc., MBA, Tami Horovitz, PhDExtracellular Vesicles and Secretome – the rise of cell-free cell-derived products
May 19, 2022
Diana Gershtein MSc., MBA, Tami Horovitz, PhD
In the last decade, the cell therapy field has matured, with some notable successes, including CAR-T therapies and gene-modified cell therapies targeting specific rare diseases. There have also been some disappointments, in particular, the failure of several MSC therapies to reach efficacy endpoints in late-stage clinical trials. In parallel, however, the realization that very many beneficial effects of cell therapy can be attributed to the cells’ secretome, has gained traction. This is evident in the exponential increase in publications relating to the secretome and extracellular vesicles (EVs), as well as in the number of start-up companies engaged in the development of such cell-free cell-derived products.
will address the following issues:
1- Enter the new kids on the block: cell-derived, cell-free products.
2- What are the advantages of EV’s and Secretome cell-free cell-derived products?
3- What are the main hurdles to be overcome when developing such a product?
5- Conclusions
Enter the new kids on the block: cell-derived, cell-free products.
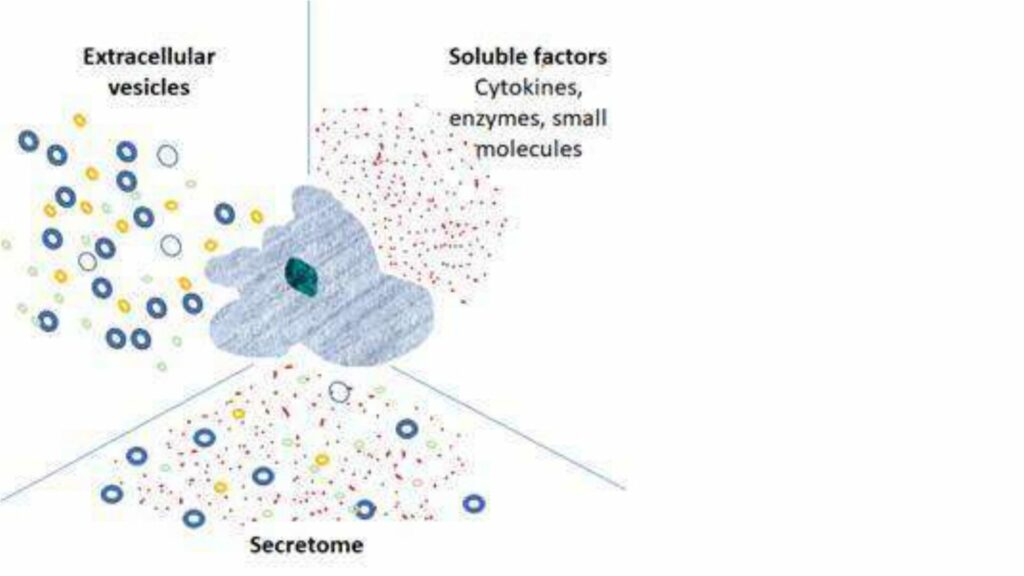
These products are derived from cell culture’s secretions – the secretome - which includes: soluble proteins and peptides, cytokines, chemokines, lipids, carbohydrates, lipid bi-layer bound vesicles including micro-vesicles, extracellular-vesicles (EVs), small EVs (sometimes referred to as exosomes) and their contents, which in addition to the above, contain nucleic acids (DNA and miRNA). The composition of the secretome depends on the cell type and microenvironmental stimuli. This is a dynamic and rich soup!
In addition, to a cell’s natural secretions, companies are engineering and/or activating cells to specifically influence the secretome by, for example, enriching for secretion of a particular factor, or directing expression of a particular factor to the surface of an EV.
What are the advantages of EV’s and Secretome – cell-free cell-derived products?
Cell-free cell-derived products may offer a number of advantages over cell therapies:
In terms of manufacturing – such a product is likely to be more stable, possibly amenable to lyophilization ,and may not require cryopreservation. This represents a huge benefit in terms of process timing, testing ,and transport logistics compared to cell therapies.
In terms of safety – a cell-free product will have no persisting cells and therefore, no potential for transformation and risk of tumor formation. Similar to traditional biological products, an EV or secretome-based product is expected to have a relatively short-term effect with half-life and clearance by predictable pathways.
In terms of efficacy - a cell-free product containing multiple active components enables a broad-spectrum approach to multiple targets, amenable for a range of indications. Repeat dosing is more likely to be required, but dosing should be easier to tailor to the patient’s requirements, based on traditional PK understandings.
What are the main hurdles to be overcome when developing such a product?
One of a kind
All cell-free cell-derived products we’ve encountered at Gsap have been very different from each other in terms of the manufacturing process, product description ,and indication. As with cell therapy, each product is unique with the manufacturing process and controls developed to suit each individual product, and careful risk assessment, is a must, to accompany each step in the development.
Cell banking
Of particular importance is the use of a well-characterized, fully tested cell bank, suitable for GMP manufacture. The cell bank is subject to the same rigorous testing requirements as required for cell therapy. The use of well-characterized commercially available cell banks, manufactured according to GMP, with a well-documented history and testing certification can be of great benefit to enable efficient translation of cell-free cell-derived products into the clinic.
Manufacturing process
Similar to cell therapy, the concept that the product is the process certainly applies here. The scientific literature is replete with examples illustrating the impact of the environment, culturing conditions, cell source, cell type, donor age, media, reagents, scale-, and vessel type, amongst other things, on cell secretome content. Early implementation of appropriate process controls is, therefore, essential to develop a process for a product sensitive to so many variables. EV or secretome-based products may be less potent than cell therapy products unless the manufacturing process can successfully concentrate the main active components whilst eliminating impurities.
No benchmarks
To date, there are no approved EV or secretome-based products approved for marketing in any territory. In contrast, the main risks from cell therapy have been established, over almost two decades of clinical use. In the absence of any benchmark products and potentially more risks, it is critical to generate an understanding for the development product’s mechanism of action (MOA) in order to successfully mitigate potential risks.
Regulatory considerations
Combined regulations are expected to apply to cell-free cell-derived products such as EVs and secrotome products, both in the US and in Europe. The exact product classification may vary, based on the product composition and attributes.
Traditional biological product regulations are expected to apply with regard to nonclinical safety testing, in other words, unlike cell therapy requirements, PK and safe pharmacology evaluation may be required and GLP safety studies may be required in two species. Safety and toxicology programs should be agreed with the regulator in advance. With cell-derived products, the potential for confounding immune response should be carefully considered.
Conclusions
These are exciting times, as biotech companies explore the use of cell secretomes for therapeutic benefit. Such cell-free cell-derived products present a whole new list of challenges for the developer and regulator alike. The new products in development are complex mixtures that require sophisticated analytical methods for their characterization – proteomics, nanoparticle tracking analysis, next-generation sequencing ,and mass spectrometry are just a few of the methods that we can expect to see more of. As with cell therapy, we trust that the individual regulatory bodies will harmonize their requirements so that development is not needlessly complicated further.
This article was prepared by both:

Diana Gershtein MSc., MBA
Advanced Therapies Section Manager

Tami Horovitz, PhD
Gsap Regulatory Submissions – Content Expert
For more information about our Pharmaceuticals industry visit: