Medical Cannabis Regulations
April 1, 2020 Sigalit Ariely-Portnoy, Ph.D, CEOMedical Cannabis Regulations
April 1, 2020
Sigalit Ariely-Portnoy, Ph.D, CEO
Topics in this article:
- The GMP requirements for Post-Harvest in Europe
- Submissions of Medical Cannabis Products in Europe
- Medical Cannabis Product Specifications (Israel , Netherlands, Germany)
1.The GMP requirements for Post-Harvest in Europe:
1.1 Medical cannabis manufacturers aiming to sell their products in Europe and specifically in the German market must meet several requirements:
• Compliance with the Herbal Medicinal Products Committee (HPMC) requirements of the European Medicines Agency (EMEA) and Good Agricultural and Collection Practice (GACP) Guidelines for Plant-Derived Starting Materials.
• Compliance with European GMP requirements, EudraLex - Volume 4 - Annex 7, Manufacture of Herbal Medicinal Products. Annex 7 (to EU GMP) presents the requirements for facility, equipment, documentation, and process control for the production of herbal products.
In the Annex, the referencing matrix is presented - for each stage of inflorescence processing, to the relevant quality requirements.
Figure No. 1: Application of Good Practices to the manufacture of herbal medicinal products:
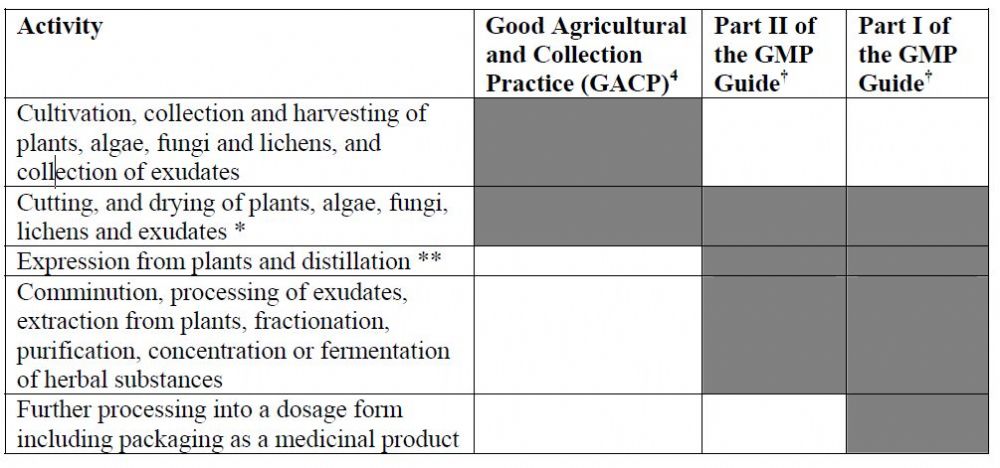
1.2 The GMP classification depends on the stage of the inflorescence processing - raw material, intermediate material, or finished product, and the possible impact on the finished product. It is the responsibility of the medical cannabis product manufacturer to ensure that the processing and production processes comply with the relevant GMP requirements.
1.3 Propagation, cultivation, and harvesting of cannabis inflorescence are subject to the GACP guidelines. The GACP guidelines do not directly fall within the GMP guidelines in the traditional sense. However, these guidelines should be the basis for establishing a proper quality assurance system.
1.4 Post Harvesting processes such as Trimming, Drying, Curing, and Hand trimming are considered as a starting material for a medical product and therefore may also be subject to EudraLex part I or part II quality requirements in addition to being subject to the GACP guidelines. Parts I and II of EudraLex Vol. 4 are intended to provide GMP guidelines to the manufacture of medicinal products and active ingredients used as raw materials, respectively. This is to ensure that they meet the quality requirements stated by them.

2.Submissions of Medical Cannabis Products in Europe
2.1 Generally, there is not a single route for Medical Cannabis approval across Europe. Each country has established its own regulations regarding Cannabis.
2.2 Cannabis is legally authorized in the Netherlands and is decriminalized for use in Germany, Portugal, the United Kingdom, the Czech Republic, Spain, and Estonia.
2.3 In countries such as France, Italy, Poland, Bulgaria, Cyprus, Denmark, Croatia, Finland, Luxembourg, Malta, Romania, Sweden, Austria, Latvia, Slovakia, Slovenia, Lithuania, Belgium, and Hungary Cannabis is still illegal.
2.4 The use of Cannabis-based medications is possible by means of three pathways (in some European countries):
• Cannabis preparations to improve wellbeing - not defined as medicine and without any specific therapeutic indication (wellness).
• A medicinal product - intended for the treatment of a labeled disease, requires clinical trials and in most cases also intended for marketing (e.g. paracetamol – analgesics).
• Medical Cannabis Path for Personal Care - For a specific patient, for certain illnesses accompanied by a doctor. This course is possible in some European countries.
2.4.1 Path No. 1: The cannabis preparations path does not require marketing authorization (MA). Cannabis preparation is originated from the Cannabis plant raw material - for example, inflorescence, oil extraction, and concentrated extraction. Cannabis preparations contain a low amount of THC (the approved level 0.3%, 0.2%) or without THC, thereby eliminating the psychoactive effect. Some countries allow the distribution without medical approval – in such countries where the sale of Cannabis is legal, or in countries where the personal use of Cannabis is decriminalized.
2.4.2 Path No. 2: Medical Cannabis for Personal Care.
This pathway allows a specific patient to receive special approval for an unapproved drug, usually under a physician’s supervision for the treatment of serious illnesses, illnesses that have not responded to other medications, or as a compassionate treatment (chronic pain, terminal cancer, or degenerative neurological disease). The doctor who provides the prescription is required to monitor and report the results of the treatment as well as any side effects or adverse events.
Medical Cannabis is supplied by pharmacies and requires EU GMP approval and a valid agreement with a local distributor. (see section 2.6).
2.4.3 Path No. 3 Cannabis-based Medicinal Products Medicinal Product - A substance or combination of substances used to cure or diagnose a disease, or to restore, repair, or alter physiological functions by activating pharmacological, immunological, or metabolic activities. This pathway requires MA for medicinal products. The MA is obtained after submission of a registration file to the health authorities, which includes clinical trials demonstrating effectiveness, efficiency, safety.
2.5 The source of the medicinal product may be synthetic or herbal:
2.5.1 A non-herbal source medicinal product route (e.g. synthetic origin). This path is a regular medicinal product route, manufactured in the pharmaceutical factories (such as paracetamol), and requires all tests to be approved (Clinical and Preclinical Trials).
2.5.4 Herbal-based medicinal product (similar to Sativex®): A medicinal product containing one or more active substances from a plant source or an herbal preparation or a combination of the two. Data should be presented that the herbal material has well-established pharmaceutical use, with over 15 years in the European community, a recognized efficacy, and an acceptable level of safety. In addition, it is necessary to demonstrate the quality and consistency of the drug manufactured in order to ensure that the patient receives the same product every time and is free of contaminants. A long tradition of using herbal medical products enables the reduction of clinical trials to prove the safety of the medicinal product. The need for pre-clinical examinations seems unnecessary, as long as the medical history of the drug and its long-standing traditional use have not caused any harm. However, the competent authorities are entitled to request, where necessary, the data for the safety assessment. In terms of the quality aspects of the medicinal product, the product must be analyzed for physical\chemical, biological, and microbiological tests and to comply with the relevant
quality standards in Europe: Pharmacopeial monograph, HPMC guidelines, and relevant directives (2004/24 / EC & 2001/83 / EC).
2.6 Obtaining a permit to import Medical Cannabis into Germany
2.6.1 Germany only imports medical Cannabis from farms and facilities operating under the 1961 Convention of Narcotic Drugs.
2.6.2 In order to import Medical Cannabis to Germany, a EUGMP certificate from the EU member state is required.
2.6.3 Manufacturers wishing to distribute Medical Cannabis products in Germany must be in agreement with or be the owner of a domestic importer. The domestic importer will have:
• Registered business.
• Wholesale distributor drug license, applied at a regional level.
• Federal-level Narcotics handling license.
2.6.4 In order to obtain the necessary licenses, importers should meet the local and federal requirements, such as: employing at least one employee responsible for narcotics, applying extensive security measures, etc.
2.6.5 Regional German inspectors have granted GMP certificates to foreign manufacturers and provided import permits to Germany.
2.6.6 The type of approval depends on the activity planned by the importer:
• Production and import authorization.
• Compliance with EU-GMP requirements.
• Approval of wholesaler-distributors.
• Compliance with Good Distribution Practice (GDP) requirements.
2.6.7 In order to obtain EU-GMP approval, an on-site audit and document set must be submitted in accordance with the requirements of the Authority.
2.6.8 The issues with the approval of Medical Cannabis as an herbal medicine in Europe are due to:
• Medical Cannabis is still included in the narcotic drug regulation in some countries.
• The difficulty in characterizing the full spectrum of the plant’s cannabinoids.
• To demonstrate product stability and purity in terms of microbiological contamination, heavy metals, and pesticides residues.
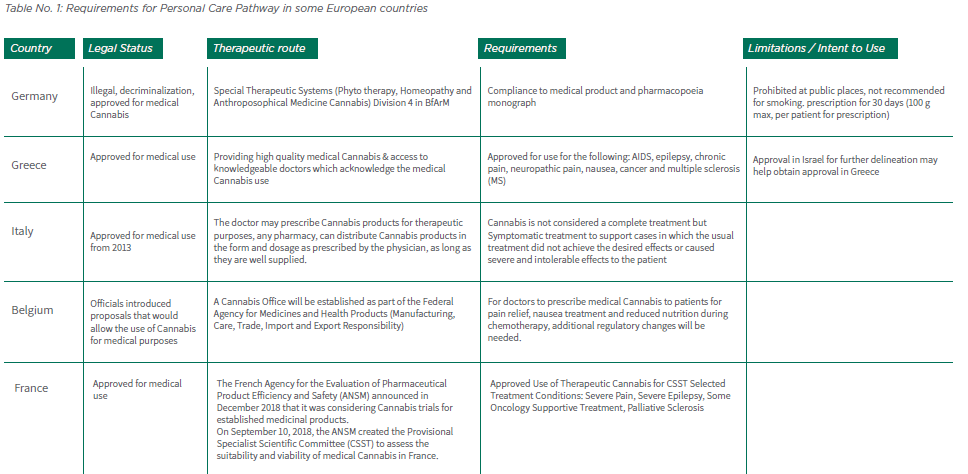
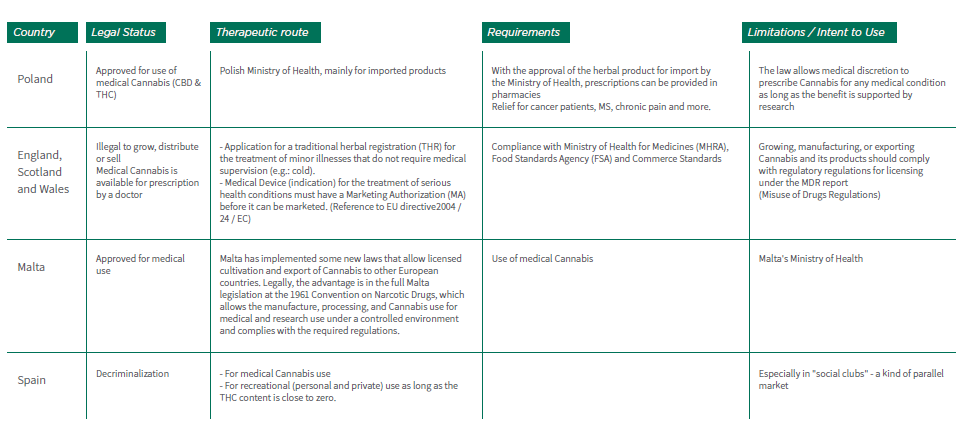
2.6.8 Table No. 1 lists the requirements for personal care in some European countries. Changes in the legal status of Medical Cannabis use through Europe and the growing demand for approval of medical Cannabis make it difficult to predict the approach to Medical Cannabis in the near future. At any rate, it is advisable to consult with the local health authorities of the country in question in order to assess if it is feasible both financially and logistically.
Table No. 1: Requirements for Personal Care Pathway in some European countries:

3.Medical Cannabis Product Specifications (Israel, Netherlands, Germany)
3.1 Similarly, to medicinal products, test methods, and specifications have been defined in some countries. The set of methods and specifications for raw material or medicinal is defined as a monograph.
3.2 In several countries where medical Cannabis products were approved, such as in the Netherlands and in Germany, local monographs for medical Cannabis were defined. We will review the defined requirements in Israel, The Netherlands, and Germany.
3.3 Israel:
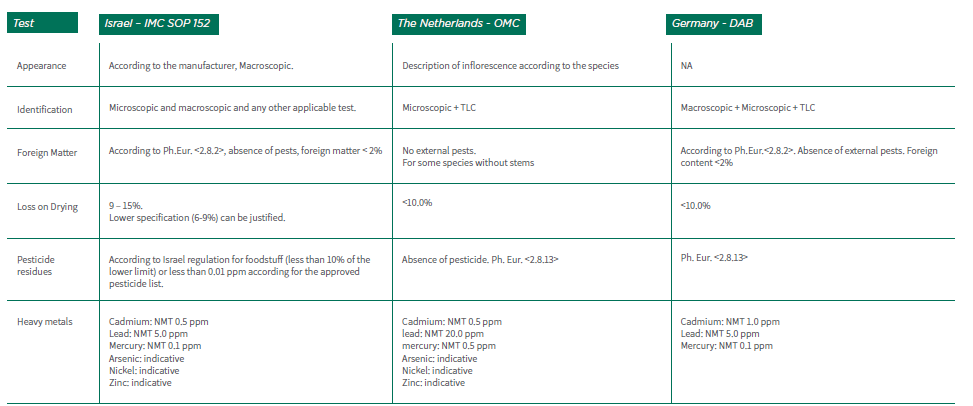
3.3.1 The Medical Cannabis unit (IMC) in the Israel Ministry of Health, has issued several guidelines related to the supply chain of Medical Cannabis. SOP 152, "IMC-GMP Quality Requirements for Manufacturing Medical Cannabis Products", defines the set of tests and specifications required for Medical Cannabis products marketed in Israel. The tests requirements described in SOP 152 are based on the requirements of the European Pharmacopoeia (Ph. Eur.) General Monograph for Herbal Drugs (Ph. Eur., 1433, Herbal Drugs).
3.3.2 According to SOP 152 and the January 2019 notification, there are four main approved medical Cannabis products groups – inflorescences, cigarettes, oils, and cookies.
3.3.3 For inflorescences (flowers or grind flowers): 12 different products are defined, which differ in the concentration of active ingredients, and for THC products also in the source
(sativa / Indica);
3.3.4 For oils: 9 different products are defined, which differ in the concentration of active ingredients.
3.4 The Netherlands:
3.4.1 The Office of Medical Cannabis (OMC) has defined specifications for 5 different inflorescences varieties that differ in the concentration of active ingredients (as of January Bedrocan (Sativa), THC concentration ~ 22%; CBD concentration <1.0%
• Bedrobinol (sativa), THC concentration ~ 13.5%; CBD concentration <1.0%
• Bediol (sativa), THC concentration ~ 6.3%; CBD concentration ~ 8%
• Bedica (indica), THC concentration ~ 14%; CBD concentration <1.0%
• Bedrolite (sativa), THC concentration <1.0%; CBD concentration ~ 9.0%
3.4.2 The Office of Medical Cannabis is also responsible for final batch release to the market.
3.5 Germany:
3.5.1 Germany has two local pharmacopoeia DAB (Deutsches Arzneibuch) and DAC (Deutscher Arzneimittel Codex). DAB has published a monograph for Cannabis inflorescence (flowers) of the female plants of Cannabis Sativa L. The requirement for Assay shall meet 90.0-110.0% of the labeled amount (calculated on a dries basis). Inflorescence storage conditions according to the monograph: tightly closed container, stored below 25°C. The monograph indicates that inflorescence products are divided into three groups: THC >> CBD, THC = CBD, THC << CBD.
3.5.2 In June 2019 a draft monograph of Cannabis oil (extract) monograph was published in DAB and it is planned to become effective during 2020. According to the draft monograph (which is still under review), the Cannabis oil should be prepared by extracting whole or grinded inflorescences of the female plants of Cannabis sativa L. The active ingredient content: THC is in the range of 1-25% and for CBD the range is NMT 10% (m/m). The active substance content shall be within the range of 90.0-110.0% of the labeled amount. The monograph includes applicable manufacturing processes: extraction (such April as ethanol or CO2), dilution of the crude extract with oil (MCT, grapes) in order to reach the target concentration. The material must undergo de-carboxylation where applicable in the process. The storage conditions according to the monograph: tightly closed container, protected from light and refrigerated (2-8 °C) – this probably will be changed in the official monograph. It should be noted that the tests specified in the German Pharmacopoeia (DAB) monographs are in addition to the requirements for Herbal Drugs (Ph. Eur.\ HPMC guidelines).
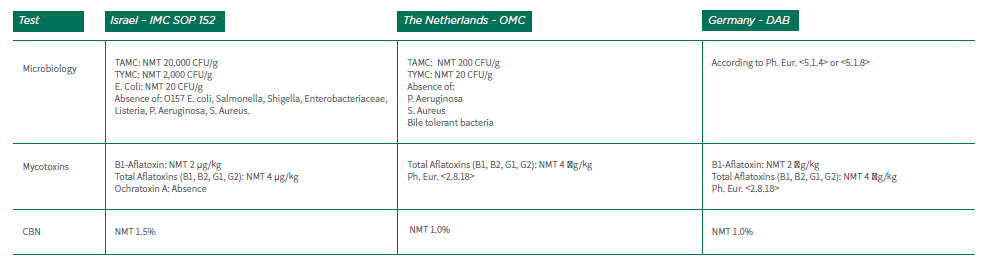
3.5.3 Table 2 includes a comparison between the specification requirements in the different countries stated above for inflorescences (flowers).
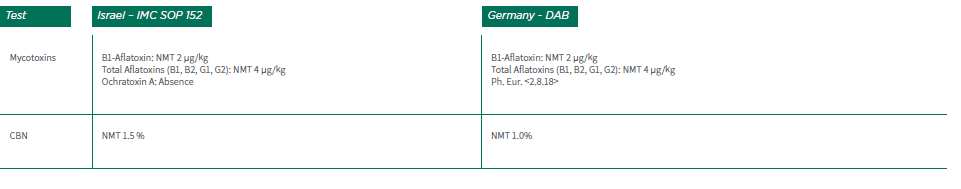
Table 3 includes a comparison for Medical cannabis oils. The tables do not include references to the active ingredient content mentioned above. Also, keep in mind that there may be differences in the test procedures between the different monographs.
Table No. 2: Medical Cannabis Inflorescence

As the world moves to implement Good Manufacturing Practices for the Medical Cannabis industry, the need for standardization is also increased. More and more countries, in Europe and in the rest of the world, are starting to initiate regulation regarding Medical Cannabis. Outside of Europe, we can find Australia, New Zealand, Brazil, and more.
Prepared by:

CEO
For more information about our services visit:




