Process Validation for Medical Devices
October 6, 2021 Jose (Yossi) Chvaicer, M.Sc.Process Validation for Medical Devices
October 6, 2021
Jose (Yossi) Chvaicer, M.Sc.
So, you want to validate your production processes. This is a great start!
There are several benefits to it as shown in this article, but…. where to begin with? How to do it? To which extent is the Validation necessary? How many samples do I need to show compliance? Not to worry, these are common questions regardless of the size of your company or your level of experience.
This article describes how to succeed in Process Validation, with real-life examples of recent Gsap projects, including the following subjects:
- Risk Management (pFMEA)
- Installation, Operational and Performance Qualifications (IQ-OQ-PQ's)
- Calibrations are not enough! - Test Method Validation (Gage R&R)
- Computer Software Validation (CSV)
- Sampling (Quantitative Methodology) - Making sense of data
In the past, most manufacturers relied on the basic IQ-OQ-PQ protocols but with time regulations became more sophisticated. The good news is that for some cases the regulatory issues can even reduce the Validation burden. Here's when Gsap will be most helpful for your company and your project.
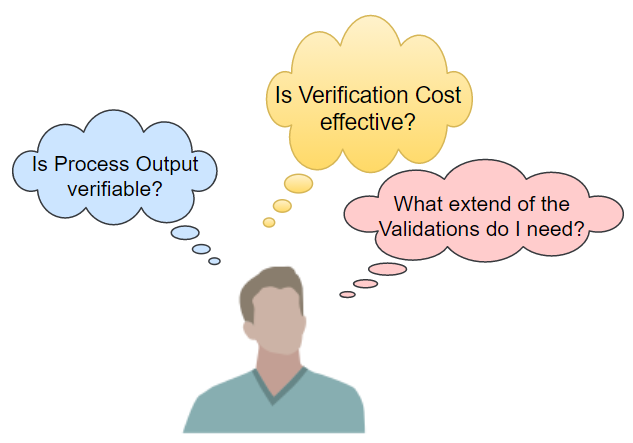
Let's begin with the FDA 21 CFR Part 820.75(a), which exempts you from Validation if you have a fully verified process. Yet, how to identify to which extend your manufacturing process is under 100% scrutiny? Is it "fully" verifiable?
Similarly, ISO 13845 releases your process from being validated where the outputs are subsequently monitored and measured. In this case, the question becomes how precise and reliable is your control over the entire process or sub-processes?
The Process Validation Guidance GHTF/SG3/ N99-10, in turn, has provided us with a helpful decision tree. It releases the manufacturer from Validation when verification alone eliminates unacceptable risks. But is verification a cost-effective solution? Again, the Validation question remains.
In practice, the Medical Device industry is generally composed of very complex manufacturing processes, sometimes involving a set of multi-part sub-processes out-sourced in many instances. So, to what extent should the Validation take in-house and outside? Still, regardless of the regulatory issues, is your customer requiring validations? What then should be the actual scope of work?
This is when you need a professional to help to decide which is the best course of action Validation will take for your business.
Gsap has experienced professionals who are aware of the different companies and their sizes, manufacturing volumes, organizational structures, and management styles. In this newsletter, we illustrate successful projects which were validated to ensure that processes operate within specifications and consistently produce safe products, all of them in compliance with the quality predetermined requirements.
One of our world-class clients has complex assembly lines for different Class II and III products. The FDA has required a confirmatory validation for all platforms. The goal was clear. Our professionals learned the processes in-depth, mapped the manufacturing flows, and developed the IQ-OQ-PQ protocols including Test Method Validation for specific continuous and discrete parameters. Five consecutive runs for each platform were analyzed, recorded and the results proved that each production line can consistently satisfy the requirements. That is to say, it was confirmed that all Device Master and History Records (DMR's and DHR's) were suitable for use in production. The project goal was achieved in time within our dedicated-for-client methodology and compliance.
Risk Management
The cornerstone preceding a successful Process Validation is the documented Risk Management file. Based on it, the Process Validation Plan indicates the actions needed to ensure that production risks, including software risks, are under control and do not violate product specifications. However, there are cases when mitigations adopted do not sufficiently cover all safety concerns for the product. As a result, residual risks become unacceptable.
We at Gsap work with what most manufacturers use for this preliminary phase: The pFMEA technique (process Failure Mode & Effect Analysis). There are three basic questions to be answered: Starting with "What can go wrong?", we then move to "What are the chances it could go wrong?", and finalize by asking " If it goes wrong what are the consequences?". The mitigations and control possibilities shall follow the assessment.
Professional support here may not only analyze and develop the Risk Management Process but also distinguish between the pFMEA and dFMEA (design FMEA). In short, Process Validation shall be effectively focused on the parameters that reflect the risks involved in your manufacturing process.

A committed Validation team is what makes the project flow.
One thing to keep in mind is to form a validation team even before the project begins. Field experience has shown that managers who gave this step the right importance had their projects flowing at a fast pace. Defining responsibilities and required deliverables from the different players is also key to the success of a Process Validation Project.
The Validation team should have representatives from at least Engineering, Quality Assurance, and Operations. Laboratories, R&D, Regulations, and Purchasing are recruited on a case-by-case basis.
The main roles of the team are:
●Early identification of the existing processes and sub-processes
●Detecting existing and potential monitoring and control features
●Approvals of the Process Validation Plan (PVP), Protocols, and Reports.
Some manufacturers call the PVP the Master Validation Plan, a document that describes the purpose, the scope and approach to be taken for the validation work, a description of the manufacturing processes, the Validation phases and associated requirements, the scheduled of work, and foreseen cases for revalidations. The MVP shall refer to relevant technical documentation, Work instructions, Sampling, and Criteria for success. In some cases, it also includes a list of tools involved in the processes.

Installation, Operational and Performance Qualifications
This is the core of Process Validation. Generally being time and work-consuming, the qualifications start with one question as well put by the PV guidance: "Is the equipment properly installed?". This is the Installation Qualification (IQ) phase of the project. The focus turns to the equipment design, installation features, cleaning requirements, environmental conditions, calibrations, safety, software, and technical documentation. This is also the phase when equipment functionality is put to prove generally off-line.
Once the infrastructure, the environmental conditions, the mechanical, electrical, and similar requirements are fulfilled, documented, and acceptable to run the process, the Installation Qualification phase is formally approved and considered finished.
Next, the attention is driven to the operational ranges which allow manufacturers to produce products inside the specifications. This is the Operational Qualification (OQ) phase. At first, a review of all process requirements including raw materials, parts, and components, assemblies, packaging, and labeling must be carefully performed. Then, a sequence of runs is planned to aim to find what should be the window of work for the parameters being evaluated. Design of Experiments (DOE) is a very useful technique to help to define operational ranges. At this phase acceptance criteria and sample size are established for both the short and long run, while the extreme values of the operational range (worst-case conditions) are determined, to comply with regulatory demands.
An innovative company has challenged Gsap to determine what parameters should be validated and what would be their levels for a successful mold injection of a Uterus Self Retaining Support (USRS) implant. The melted material was extremely expensive so that the runs needed to be precise. Decisions for trials were taken beforehand based on a very high professional knowledge. The validation team decided that Holding Pressure and Cooling time should be challenged at two levels each, namely High and Low. A methodical DOE took place outcoming the window of work for the acceptable extreme values. The recommended levels were successfully determined for the next phase, the Performance Qualification. Three batches, all within the recommended levels, concluded the PQ.
The example above was completed under 100% acceptance of the items produced. The summary report emphasized the need for monitoring both the cooling time and holding pressure for runs at normal operating conditions.
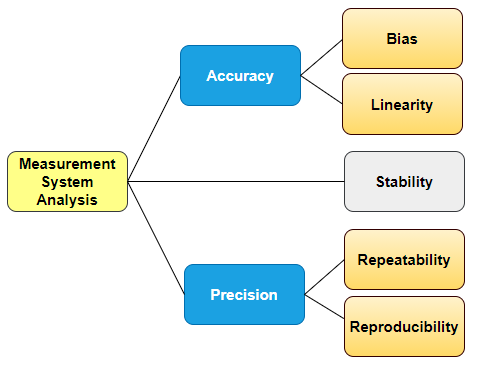
Calibrations are not enough! - Test Method Validation (Gage R&R)
Yes, this is correct. We all know process instruments must be calibrated, but calibration alone will not suffice inspections. It sounds weird, even fastidious, yet it is easy to understand. Suppose that a part that is produced at high volumes has a Critical to Quality (CTQ) dimension. This variable is manually measured along with production, in three different shifts, by one calibrated micrometer.
Regulations require that the measurement method produces valid results, regardless of the instrument used, variable, or attribute data alike. Back to our example, if we were up to measure the same part at those three shifts, we would expect to get three different results. The theoretical difference might not be significant, but can you prove it? Let's be aware that the tolerances are not of concern here. At times, you will be required to present supporting documentation showing that measurements or testing methods do not vary more than 10% over shifts and operators, regardless of acceptance. In other words, variations in results should be mostly due to the difference among parts and not vary due to humans or any other factor over ranges and time.
Likewise, for a large staff, an agreement between different QC personnel may as well be questionable.
This is the so-called Test Method Validation (TMV) where the Repeatability and Reproducibility of testing and measurement methods (Gage R&R) are used.
TMV is an integral part of any successful validation for manufacturing processes. Again, our professional support in developing correct protocols and executing them properly will help you overcome the TMV challenge.
Computer Software Validation
If your product has software applications associated with it, you are required to confirm that the software specifications conform to the intended use of the product and they fit the user's needs. Unless there is an in-house specialist on Software Validation this is definitively the place to outsource support.
Electronic Records and Signatures, data integrity and workflow, accessibility, audit trail, and retrieval capabilities are a few of the qualifications needed.
A Bio-Tech company in the cell-growing for blood disorder market had a large number of QC lab devices, each with its own dedicated software application, turned to Gsap for Software Validation support. Their product was stored at deep freeze. The analysis performed by each device, the results, and the generated reports for batch release were backed up in the cloud. Besides the qualifications needed for all temperature conditions, cyber protection for data became a must. But how to validate software that is working only with cloud-generated files? We started with a GAP Analysis for the computerized Systems for each device. Next, the work focused on a Criticality Assessment and Software Installation issues. Finally, we approached Configurations, Security Measures, Remote Storage, and Retrieval features, Data Work Flow, Data Integrity, Audit trails, Readability, and Disaster recovery. As a result, qualifications have fulfilled the requirements not only for the 21 CRF Part 11 but also for the Annex 11 of the Eudralex EC Public Health and Risk Assessment. ALCOA+ requirements were also covered.
Making sense of data
We found a way to cope with one of the fear-most requirements: Statistical techniques. It is easier to think of it as self-defense for your project. In this way, the sampling rationale becomes even helpful.
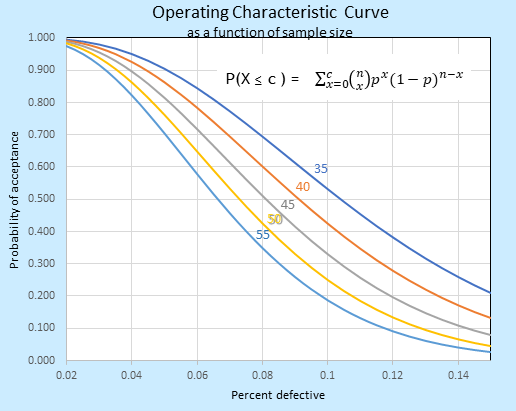
Suppose you have adopted the ANSI Z1.4 for inspections. Now you can state the purpose of the acceptance plan, how much is routinely accepted 95% of the time, how much is expected to be rejected, and so on. Tables and Operational Characteristic curves (see below) are of easy access and ready for your ease. It seems everything is Ok.
But the ANSI is mostly used for lots that have been already produced. It looks to the past. What happens if you are going to validate a batch to be produced in the future? How to decide which is the appropriate success criterion looking to the future? You will need to assume a standard deviation of the upcoming sampling and hope variation does not exceed it. Well, how big must the sample size be for a high degree of confidence? What reliability do you desire for your product? After all, decisions are based on a sample. What happens if there are multiple runs and different batches? Do they share homoscedasticity (common variance)? The answers are not so simple.
Cp and Cpk indexes are common ways to validate manufacturing by simply proving the capability of a process. However, they require samples to be originated from a normally distributed population, an issue frequently overlooked. Can you prove the normality of your data? How many samples do you need for a minimum Cpk of 1.33? Are there outliers? What to do with them? How to interpret the p-value obtained? These are some of the questions concerning the statistical side of Validations.
Our support starts by analyzing results. If they do not come from a normally distributed population, we will search for the appropriate distribution to work with it. Occasionally data transformation may be of help. Those issues are important to decide the overall validity of the analysis. They require some expertise, but whatever the case is, we will help you comply with the statistical requirements, select the correct sample size, make sense of data, and feel comfortable with the numbers.
Benefits and why is Process Validation so important?
First of all, because it can be enforced on you. It's the law. The Code of Federal Regulations (CFR) are not recommendations but rules that must be followed.
Additionally, there are several advantages for validating manufacturing processes in the medical device industry.
●It ensures your equipment operates correctly and is maintained properly. This means immediate savings, less downtime, and product quality benefits.
●In the long run, it reduces inspection and correction costs. Validated processes shall be monitored and controlled so that your production and products are safe and reliable over time.
●Processes are understood by the staff. It makes good business sense when you and your staff comprehend and control the processes. A knowledgeable staff means your business is competitive and ready to cope with any of the day-by-day challenges.
Validated processes under regulatory compliance shall be one of the ultimate goals for your business. This may be the right moment to improve existing Validation procedures, or if you are new on the subject, to correctly introduce Validation, alongside professional help.
Improving your staff's capabilities for the development and execution of qualifications is also a way to go with Gsap seminars and courses. For more information, please contact us.
Now is your turn.
This article was prepared by:

Jose (Yossi) Chvaicer, M.Sc.
Senior Validation and Quality Engineer
For more information about our validation services visit: