Gsap CRO
End-to-end clinical trials management
At Gsap CRO, we are committed to driving success in clinical studies by providing comprehensive, end-to-end solutions backed by unmatched expertise at every phase of the process. Our team of seasoned clinical research professionals delivers unparalleled support, ensuring that your trials meet and exceed expectations. We’re more than just a CRO; we’re a trusted partner and advisor, dedicated to helping every project achieve its full potential.
Need guidance with your clinical project? Gsap CRO experts are ready to support your project.
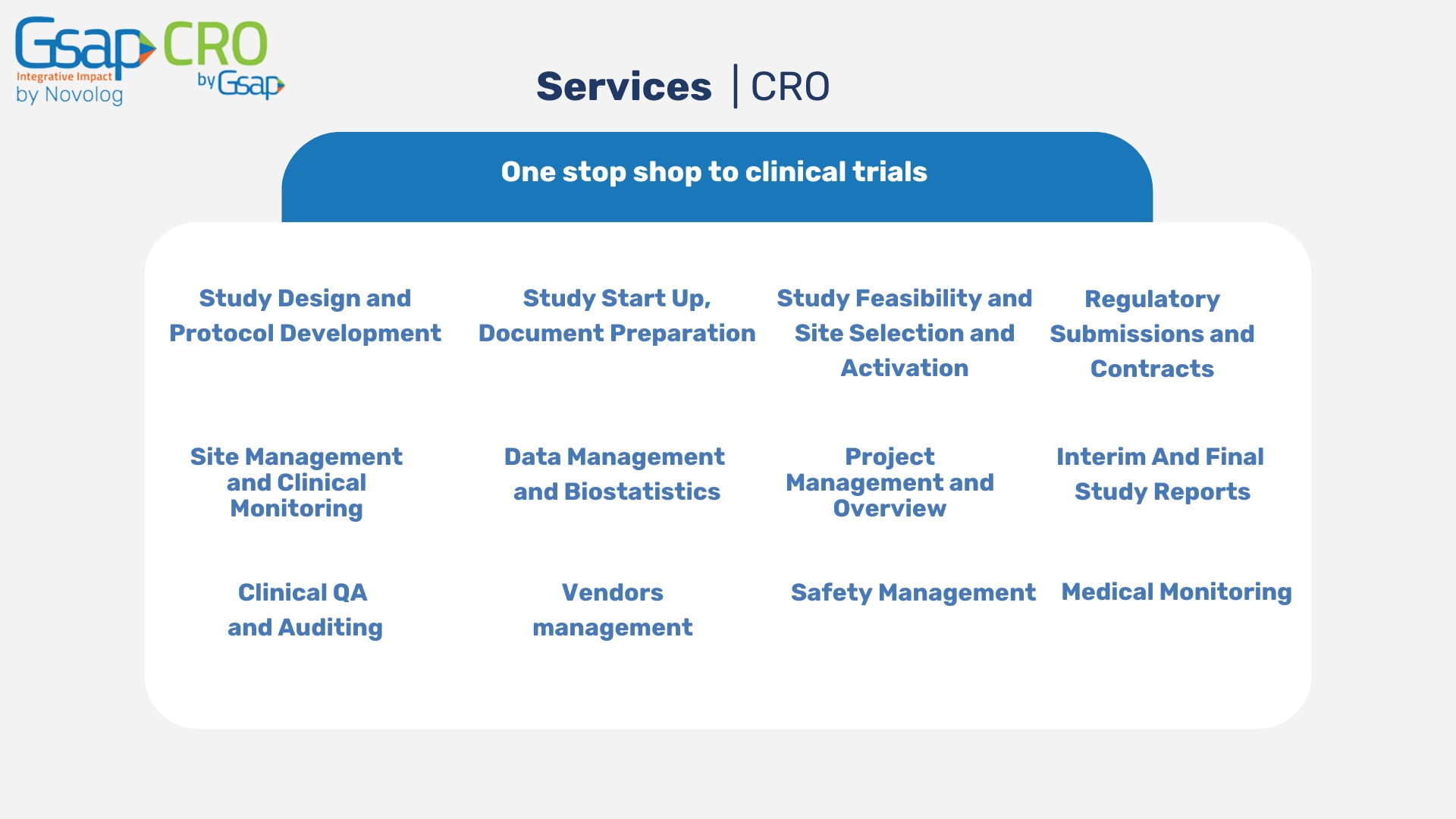
Services
Study design and Protocol development
We study your product from the scientific and practical perspective, intended use, indication and population, regulatory environment as well as your broader strategy and resources to tailor the study design to your needs and objectives. We enhance your study design to optimize subject recruitment, retention, and satisfaction.
Study start up, document preparation
We have a vast knowledge of the most updated clinical study regulations. We follow our SOPs and all relevant regulations to deliver appropriate content and comprehensive documents that are in line with the requirements and study needs.
We professionally develop all the core documents required for submissions and study conduct i.e.: Protocol, ICF, IFU, IB etc.
Study Feasibility and Sites selection and activation
Following a thorough understanding of your study characteristics and needs, we reach out to our network of sites to identify the most suitable sites and Investigators for successful and effective study execution.
Regulatory submissions and contracts
We plan and complete the submission package according to the local regulatory requirements, and communicate with the Ethics Committees Regulatory Authorities, and Regulatory Agencies to obtain all required approvals. We negotiate financial contracts with the clinical sites to full execution.
Site management and Clinical Monitoring
We build strong relations with the site staff for optimal cooperation. We devise a source document verification (SDV) strategy to monitor data validity, ensure compliance with protocol and regulations and that subject safety and rights are respected
Data management and Biostatistics
We are responsible for the quality of your data, we develop the case report form (CRF), data management plans and guidelines, electronic data capture (eDC) setup, design, verification, and validation. We manage interim analyses, database locks, and biostatistics until the final study report.
Project management and Overview
We manage your project in close collaboration and partnership with all parties and vendors, with respect to timelines, milestones, training materials, sites support, in-house monitoring, open action follow-up, eTMF, Clinical Management Plan (CMP), and internal SOP. This approach ensures a high level of project management and quality of study conduct.
Interim and final study reports
From the early stages of the study, we already consider your broader needs and strategy for the final study report. We place particular importance on writing the study report as the final document which summarizes the entire clinical operation and study results. We perform this diligently and professionally according to high industry standards
Clinical QA and Auditing
We examine trial-related activities and documents to determine compliance to the protocol, sponsor’s Standard Operating Procedures (SOPs), Good Clinical Practice (GCP), and the applicable regulatory requirements. Provide various QA services including; development of processes, SOPs, preparation, and auditing.