The minimum QMS for Design and Development
September 12, 2022 Ms. Silvana Singer AtiasThe minimum QMS for Design and Development
September 12, 2022
Ms. Silvana Singer Atias
Creating a medical device requires you to concentrate on the process of documenting and prototyping phases. In highly regulated areas such as healthcare, a quality management system (QMS) is crucial to the development of a new product.
The question is what is the minimum QMS for the design and development stage? To answer this question, we recommend considering the following:
A medical device should be safe and effective to reduce product recalls and revisions, demonstrate compliance with all relevant regulatory standards, and be commercialized quickly.
This article will address:
1- General Recommendations for QMS needed at the development stage for medical device products
2- The bare minimum required QMS documents
3 - Conclusion
General recommendations for QMS needed at the development stage for medical device products:
● Documentation should demonstrate consistency, reproducibility, and repeatability. Furthermore, documentation should communicate what to do, when to do it, and how to do it effectively.
●You should ‘right-size’ your QMS as you build it. In other words, the size of the QMS should reflect the size of your organization. By ‘right-sizing’ your QMS, you ensure that you build the quality procedures you need at the right time. The needs of a 5-employee company differ from one of 50 employees and from 500 employees.
●You need to make sure you consider the product realization and the lifecycle of the device. Identify all the documents that you will be required to generate as part of these processes.
●Be sure to determine who is the owner of each document, for how long it must be kept, and where it will be stored before you begin documenting anything.
●Consider the availability of documentation and an effective and efficient way of managing the documentation. Medical device companies are strong when they manage their documentation well.
We recommend using an Electronic Quality Management System (eQMS). If you choose eQMS, you need to define your documentation and record control with your eQMS.
●Developing and updating your document management strategy takes a different approach as your company becomes more established. It is advisable to break down your document management system into phases and plan each phase carefully.
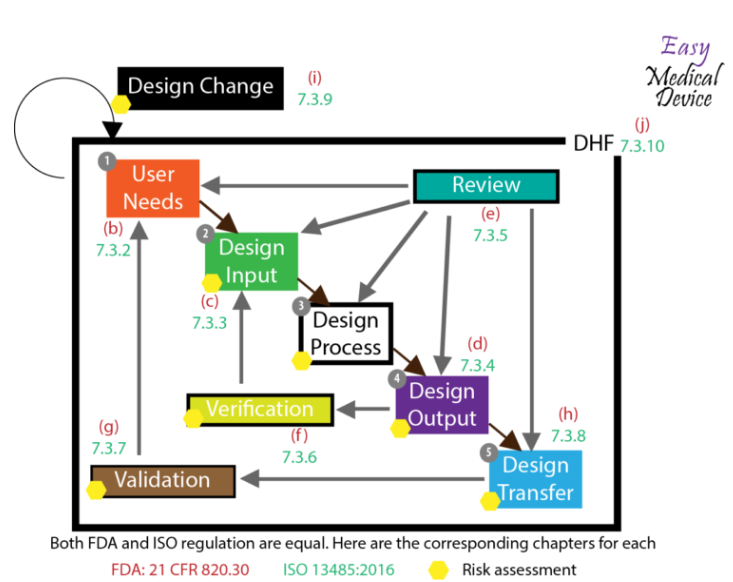
The Bare minimum required QMS documents:
(1) Regulatory Strategy Document
The regulatory strategy includes product description, product intended use and indication for use, product classification, regulatory pathway per relevant markets, applicable product verification and validation tests, list of applicable standards and guidance
(2) Design and Development Control Procedure
| The design and development control procedure describes the method for initiating, controlling, and documenting product design processes. |
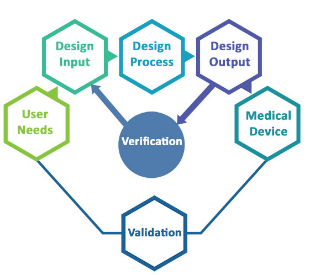
It is recommended that the procedure address the following processes:
●Processes for defining the user needs, product value proposition, and VOCs (Voice of Customer),
●Processes for defining product specification (design inputs),
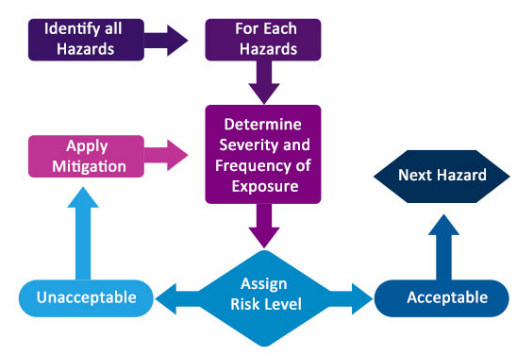
●Risk Management processes in accordance with ISO 14971:2019 including failure mode analysis
●Guidelines for defining Sample size and rationale for design validation and process validation processes,
●Processes for design verification and design validation
●Processes for design reviews:
●DIR (Design input review), DOR (Design Output review), Verification & Validations, Statistical Techniques, Traceability.
(3) Purchasing Controls Procedure
Purchasing control procedure includes processes to facilitate supplier management, guidelines to oversee the purchase of materials, components, products, or services according to quality standards, and conformance to all specified requirements. These shall include receiving inspection processes and control of purchasing records.
(4) Document and Record Control
This is one of the most important procedures for the design and development phase. Many manufacturers are not aware of how critical it is to document the design at the very early stages of design and development. This includes a methodology for numbering and traceability of all documentation and records that are created from the very beginning of the design and development (such as design inputs, design outputs, test protocols, any test results, reports, design reviews, and any changes to the design, risk management file).
Document and record control procedure means defining a formal document control process from numbering documents and records to adding/maintaining/changing/removing documents and records.
(5) Engineering Change Orders (ECOs)
ECO is an important tool that ensures that changes that are done to a product(s) under development are identified, reviewed, validated/verified, documented, and approved before their implementation. It is highly recommended to define the ECO process for stages before design freeze (or design output review) and after design freeze. Changes before the design freeze shall include as a minimum: Details of the change, the reason for the change, and the approver of the change. All records must be kept in all phases.
(6) Quality Manual
A full quality manual is not required at the early stages of the design and development, yet a frame with the building bloc needs to be defined for the quality manual.
This frame needs to include the scope of the quality management system and its exclusions, a list of the planned documented procedures for the quality management system, and a cross-reference table to later include the relationships between the QMS procedures and the regulatory requirements.
It is also advisable to include in the quality manual the company’s organizational structure, specifying the functions in the organization.
Conclusion
At the beginning of the development of a new product, it is necessary to plan for the evolution, including the minimum documentation required at this step, thinking about the right size and procedures of the QMS.
Good luck!
This article was prepared by:

Ms. Silvana Singer Atias
RA & QA Project Manager Gsap, Medical Device




