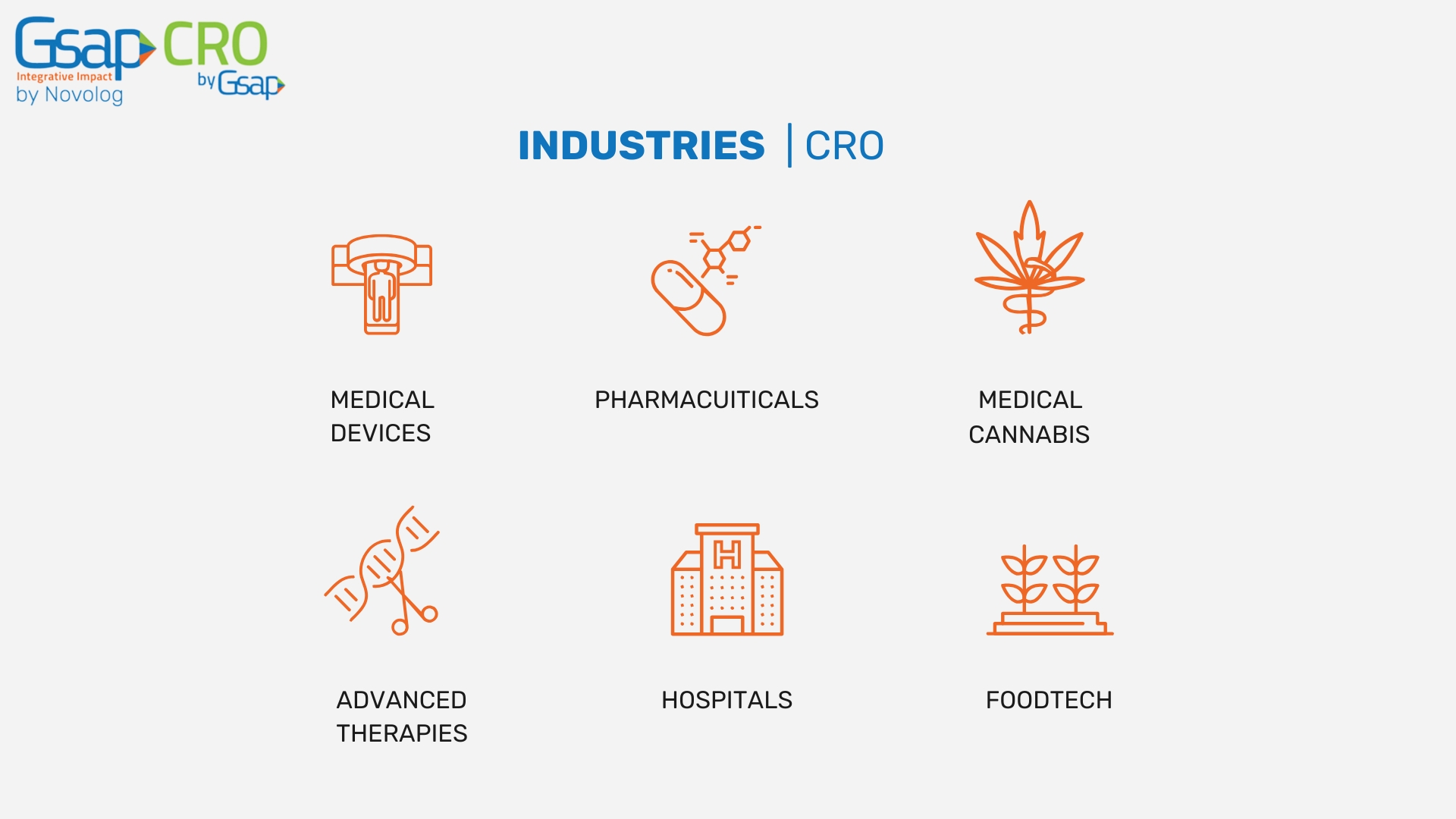
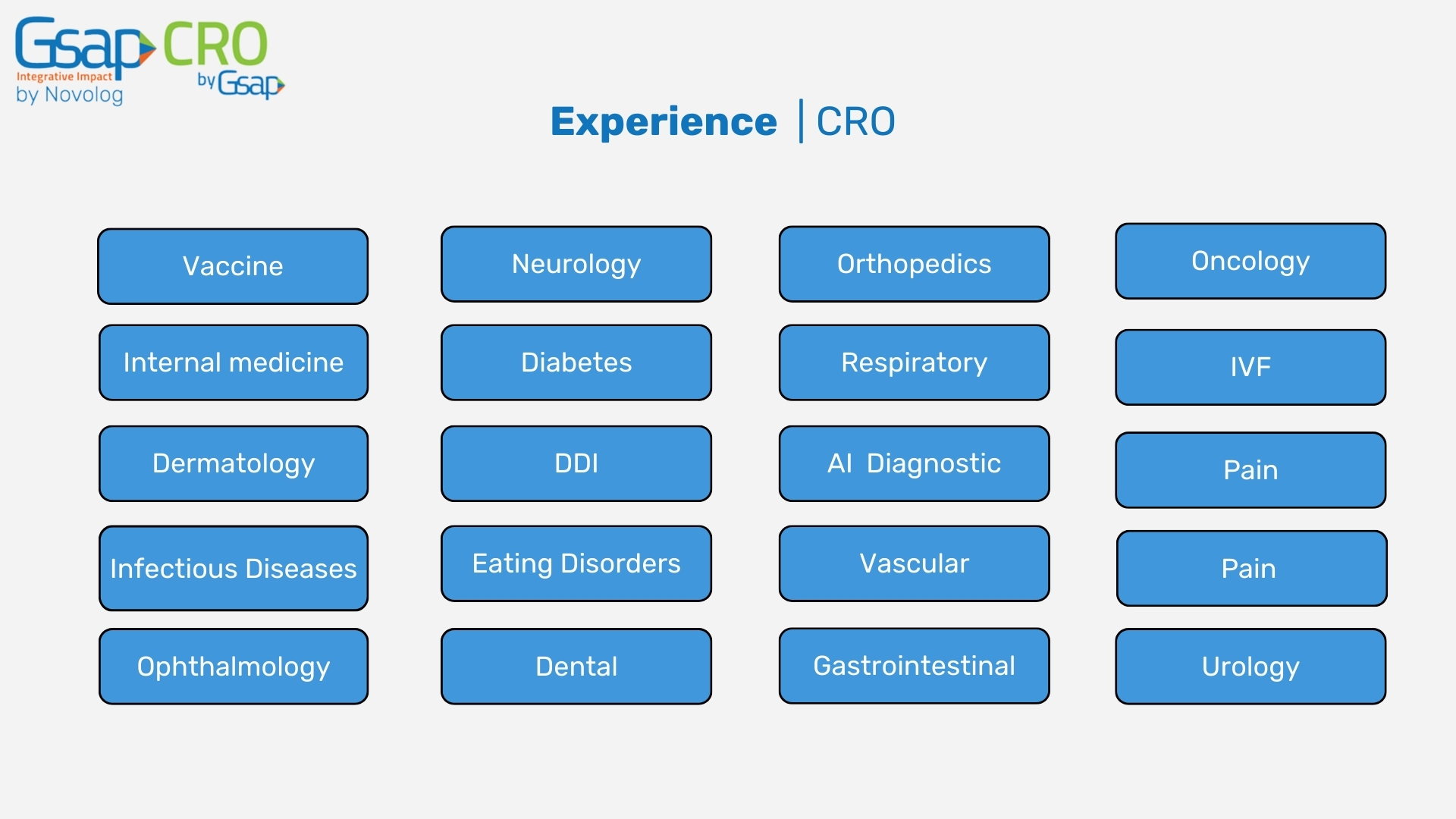
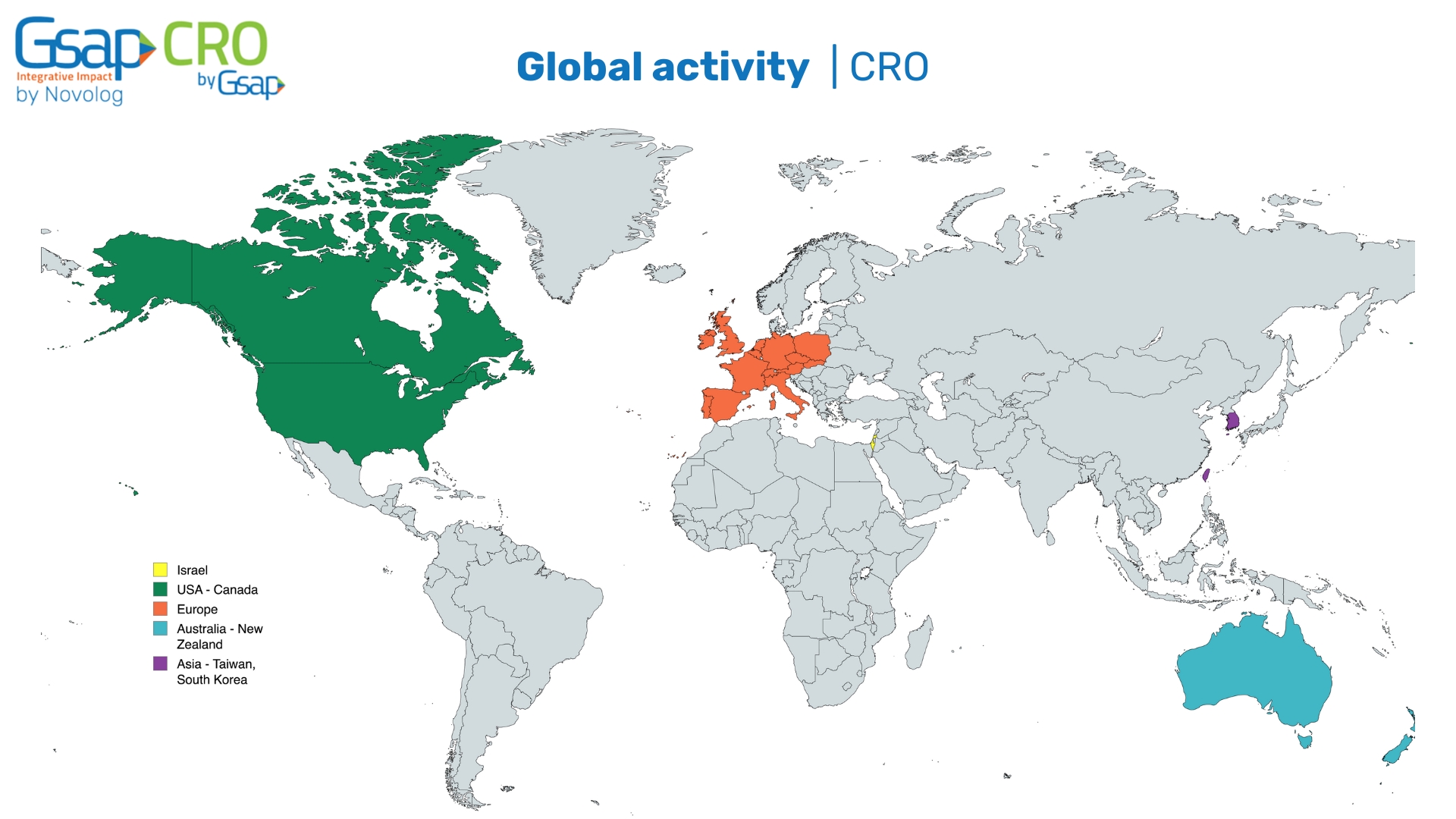
At Gsap CRO, we are proud to offer clinical research support on a global scale. Our international presence allows us to provide comprehensive clinical trial management and regulatory expertise across various regions. By working closely with our partners around the world, we help healthcare companies navigate the complexities of clinical research in different regulatory environments. Whether your clinical trial is based in North America, Europe, or Asia, our global activity ensures compliance with local regulations while maintaining the highest standards of quality and safety.
Our global operations enable us to support clinical trials in key regions, bringing innovation to market faster and more effectively.
Our International Activity
We have an extensive global presence, actively managing clinical trials in the following key regions:
- Israel
- USA & Canada
- Europe
- Australia & New Zealand
- Asia (Taiwan & South Korea)
Contact US
For more information on our global activity or to discuss how Gsap CRO can support your international clinical trial needs, please contact us:
+972(04)-6404300